We expect emerging data to create significant momentum as we strive to deliver value for patients and shareholders
ADVANCING INNOVATION
Thoughtfully Approaching GI Drug Discovery
Ironwood Pharmaceuticals’ commitment to redefining the standard of care for patients suffering from GI diseases and disorders is unwavering. We understand that debilitating GI diseases exist, and countless patients need meaningful and effective medicines.
We are focused on following the science and strengthening our portfolio by pursuing opportunities in organic GI diseases with significant unmet medical need, strong mechanistic rationale, and commercial viability. By following this strategy, we can leverage our leading GI capabilities and expertise now and in the future.
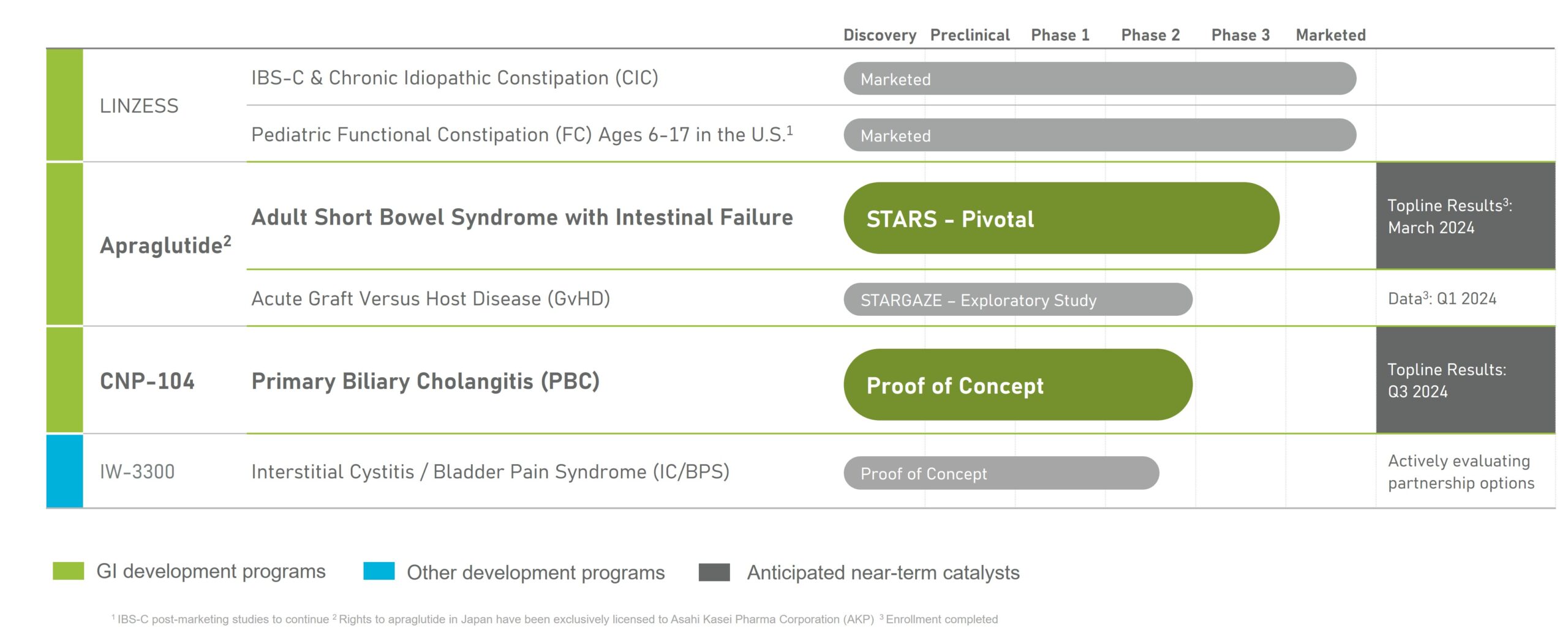
Strengthening Our Leading GI Portfolio
Apraglutide
In June 2023, we expanded our pipeline through the acquisition of VectivBio to include apraglutide, an investigational GLP-2 analog being evaluated as a potential once-weekly treatment for patients with Short Bowel Syndrome with intestinal failure (SBS-IF).
SBS-IF is a severe malabsorptive condition requiring ongoing I.V. administration of fluids and nutrients and is associated with significant morbidity and mortality, high economic burden, and an impaired quality of life. A substantial number of SBS-IF patients remain dependent on chronic parenteral support, and there is considerable unmet need in this patient population, which has an estimated addressable population of 18,000 adult patients across the U.S., Europe, and Japan
CNP-104
In November 2021 into a collaboration and license option agreement with COUR Pharmaceuticals Development Company, Inc., which grants us an option to acquire an exclusive license to research, develop, manufacture and commercialize, in the U.S., products containing CNP-104, a potential treatment for PBC, a rare autoimmune disease targeting the liver that affects an estimated 130,000 people in the U.S. according to a study published in Gastroenterology. Currently, there is no approved therapy that addresses the root cause of the autoimmune destruction of the bile ducts in PBC. In December 2021, COUR received U.S. Fast Track Designation. The proof-of-concept study is ongoing, and given the strong science underlying this therapy, we plan to assess T-cell response in patients dosed with CNP-104 in the second half of 2023, which will inform the timing of topline data.
IW-3300
We are developing our wholly owned IW-3300 – a GC-C agonist - for the potential treatment of visceral pain conditions, such as interstitial cystitis/bladder pain syndrome (IC/BPS) and endometriosis. IC/BPS affects an estimated 4 to 12 million Americans, according to the Interstitial Cystitis Association. An estimated 4 million reproductive-age women in the U.S. have been diagnosed with endometriosis, according to a study published in Gynecologic and Obstetric Investigation. Both diseases have a limited number of treatment options available. In 2022, we successfully completed dosing studies in healthy volunteers, and are currently executing the proof-of-concept study in IC/BPS. IC/BPS patients are being screened, dosed and site activations are going very well.


